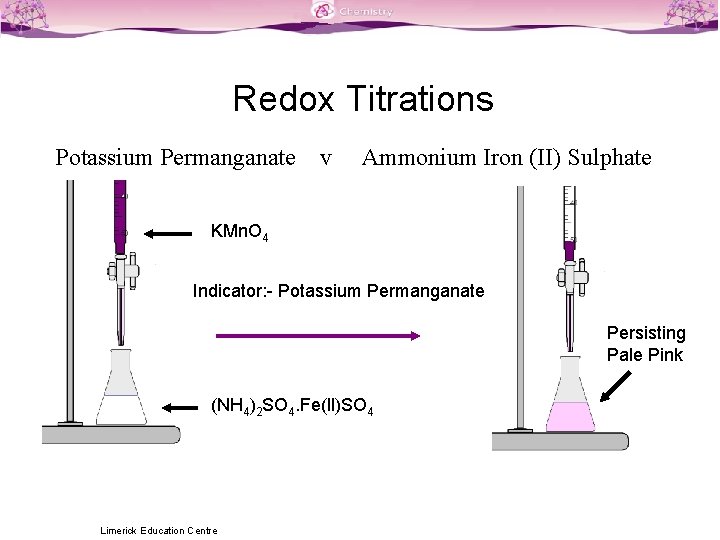
Titration Formula Class 12 . Write a balanced equation for the titration reaction. The reaction between kmno 4 and oxalic acid is a redox reaction and the titration is therefore called a redox titration. Acidimetry, alkalimetry, indicator, end point, equivalence point. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. Introduction to titration and it types. The base has a hydroxide. A titration can define the volume of one solution required to react correctly with an identified volume of a different solution. It is an inorganic light green coloured. The titration formula calculates the concentration of an analyte by multiplying the normality (n) of the titrant by its volume (v). Ammonium ferrous sulphate or ammonium iron (ii) sulphate is called mohr’s salt. (redox reactions) the oxidation and reduction reactions in aqueous solutions involve the transfer of electrons from. The reactants are sodium hydroxide (\ (\text {naoh}\)) and sulfuric acid (\ (\text {h}_ {2}\text {so}_ {4}\)).
from slidetodoc.com
The reaction between kmno 4 and oxalic acid is a redox reaction and the titration is therefore called a redox titration. Introduction to titration and it types. The base has a hydroxide. (redox reactions) the oxidation and reduction reactions in aqueous solutions involve the transfer of electrons from. Write a balanced equation for the titration reaction. The reactants are sodium hydroxide (\ (\text {naoh}\)) and sulfuric acid (\ (\text {h}_ {2}\text {so}_ {4}\)). Ammonium ferrous sulphate or ammonium iron (ii) sulphate is called mohr’s salt. A titration can define the volume of one solution required to react correctly with an identified volume of a different solution. Acidimetry, alkalimetry, indicator, end point, equivalence point. The titration formula calculates the concentration of an analyte by multiplying the normality (n) of the titrant by its volume (v).
Titration Colour Changes SLSS Science Limerick Education Centre
Titration Formula Class 12 It is an inorganic light green coloured. The base has a hydroxide. The reactants are sodium hydroxide (\ (\text {naoh}\)) and sulfuric acid (\ (\text {h}_ {2}\text {so}_ {4}\)). A titration can define the volume of one solution required to react correctly with an identified volume of a different solution. It is an inorganic light green coloured. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. Write a balanced equation for the titration reaction. (redox reactions) the oxidation and reduction reactions in aqueous solutions involve the transfer of electrons from. The reaction between kmno 4 and oxalic acid is a redox reaction and the titration is therefore called a redox titration. Ammonium ferrous sulphate or ammonium iron (ii) sulphate is called mohr’s salt. The titration formula calculates the concentration of an analyte by multiplying the normality (n) of the titrant by its volume (v). Introduction to titration and it types. Acidimetry, alkalimetry, indicator, end point, equivalence point.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Titration Formula Class 12 The titration formula calculates the concentration of an analyte by multiplying the normality (n) of the titrant by its volume (v). The reaction between kmno 4 and oxalic acid is a redox reaction and the titration is therefore called a redox titration. Introduction to titration and it types. Acidimetry, alkalimetry, indicator, end point, equivalence point. (redox reactions) the oxidation and. Titration Formula Class 12.
From www.studocu.com
Class XII Chemistry 202223 Experiment No Oxidation Reduction Titration Formula Class 12 A titration can define the volume of one solution required to react correctly with an identified volume of a different solution. It is an inorganic light green coloured. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. Ammonium ferrous sulphate or ammonium iron (ii) sulphate is called mohr’s salt. Introduction to titration and. Titration Formula Class 12.
From fity.club
Titration Formula Titration Formula Class 12 The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. Introduction to titration and it types. (redox reactions) the oxidation and reduction reactions in aqueous solutions involve the transfer of electrons from. A titration can define the volume of one solution required to react correctly with an identified volume of a different solution. The. Titration Formula Class 12.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Formula Class 12 The reactants are sodium hydroxide (\ (\text {naoh}\)) and sulfuric acid (\ (\text {h}_ {2}\text {so}_ {4}\)). The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. It is an inorganic light green coloured. Acidimetry, alkalimetry, indicator, end point, equivalence point. Introduction to titration and it types. Write a balanced equation for the titration. Titration Formula Class 12.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Formula Class 12 Introduction to titration and it types. The reactants are sodium hydroxide (\ (\text {naoh}\)) and sulfuric acid (\ (\text {h}_ {2}\text {so}_ {4}\)). The base has a hydroxide. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. A titration can define the volume of one solution required to react correctly with an identified. Titration Formula Class 12.
From boardtopper.blogspot.com
Titration 2 Class 12 CBSE Practical All Study Guide at one Place! Titration Formula Class 12 Acidimetry, alkalimetry, indicator, end point, equivalence point. The titration formula calculates the concentration of an analyte by multiplying the normality (n) of the titrant by its volume (v). Ammonium ferrous sulphate or ammonium iron (ii) sulphate is called mohr’s salt. Introduction to titration and it types. The reactants are sodium hydroxide (\ (\text {naoh}\)) and sulfuric acid (\ (\text {h}_. Titration Formula Class 12.
From fity.club
Titration Calculations Titration Formula Class 12 The titration formula calculates the concentration of an analyte by multiplying the normality (n) of the titrant by its volume (v). Acidimetry, alkalimetry, indicator, end point, equivalence point. Introduction to titration and it types. It is an inorganic light green coloured. The reactants are sodium hydroxide (\ (\text {naoh}\)) and sulfuric acid (\ (\text {h}_ {2}\text {so}_ {4}\)). The titration. Titration Formula Class 12.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration Formula Class 12 It is an inorganic light green coloured. Ammonium ferrous sulphate or ammonium iron (ii) sulphate is called mohr’s salt. A titration can define the volume of one solution required to react correctly with an identified volume of a different solution. Acidimetry, alkalimetry, indicator, end point, equivalence point. The base has a hydroxide. The reactants are sodium hydroxide (\ (\text {naoh}\)). Titration Formula Class 12.
From www.youtube.com
Conductometric Titrations Electrochemistry L11 Class 12 Chemistry Titration Formula Class 12 The reaction between kmno 4 and oxalic acid is a redox reaction and the titration is therefore called a redox titration. Introduction to titration and it types. It is an inorganic light green coloured. The base has a hydroxide. Ammonium ferrous sulphate or ammonium iron (ii) sulphate is called mohr’s salt. A titration can define the volume of one solution. Titration Formula Class 12.
From annbjordanxo.blob.core.windows.net
Titration Chemistry Practice Problems Titration Formula Class 12 A titration can define the volume of one solution required to react correctly with an identified volume of a different solution. Ammonium ferrous sulphate or ammonium iron (ii) sulphate is called mohr’s salt. The reaction between kmno 4 and oxalic acid is a redox reaction and the titration is therefore called a redox titration. (redox reactions) the oxidation and reduction. Titration Formula Class 12.
From www.pinterest.com.au
titration process and its application Chemistry practical, Teaching Titration Formula Class 12 It is an inorganic light green coloured. Introduction to titration and it types. Write a balanced equation for the titration reaction. (redox reactions) the oxidation and reduction reactions in aqueous solutions involve the transfer of electrons from. Acidimetry, alkalimetry, indicator, end point, equivalence point. A titration can define the volume of one solution required to react correctly with an identified. Titration Formula Class 12.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Formula Class 12 (redox reactions) the oxidation and reduction reactions in aqueous solutions involve the transfer of electrons from. The reaction between kmno 4 and oxalic acid is a redox reaction and the titration is therefore called a redox titration. Ammonium ferrous sulphate or ammonium iron (ii) sulphate is called mohr’s salt. A titration can define the volume of one solution required to. Titration Formula Class 12.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Formula Class 12 It is an inorganic light green coloured. Introduction to titration and it types. A titration can define the volume of one solution required to react correctly with an identified volume of a different solution. The titration formula calculates the concentration of an analyte by multiplying the normality (n) of the titrant by its volume (v). Acidimetry, alkalimetry, indicator, end point,. Titration Formula Class 12.
From www.youtube.com
Class 12 Titration Easy way of doing TITRATION and tricks to Titration Formula Class 12 Introduction to titration and it types. (redox reactions) the oxidation and reduction reactions in aqueous solutions involve the transfer of electrons from. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The base has a hydroxide. Write a balanced equation for the titration reaction. A titration can define the volume of one solution. Titration Formula Class 12.
From chemistry.analia-sanchez.net
Titration Notes Chemistry Classes / Ronald Reagan S.H.S. Titration Formula Class 12 The titration formula calculates the concentration of an analyte by multiplying the normality (n) of the titrant by its volume (v). Introduction to titration and it types. The reaction between kmno 4 and oxalic acid is a redox reaction and the titration is therefore called a redox titration. The titration of potassium permanganate (kmno 4) against mohr salt is an. Titration Formula Class 12.
From www.youtube.com
Electrochemistry Class 12 Chemistry Kohlrausch's Law and Titration Formula Class 12 Write a balanced equation for the titration reaction. The base has a hydroxide. A titration can define the volume of one solution required to react correctly with an identified volume of a different solution. Acidimetry, alkalimetry, indicator, end point, equivalence point. The reaction between kmno 4 and oxalic acid is a redox reaction and the titration is therefore called a. Titration Formula Class 12.
From adawyaf.blogspot.com
Chemistry Grade 12 , Activity 2 , Titration Experiment Titration Formula Class 12 Acidimetry, alkalimetry, indicator, end point, equivalence point. The reactants are sodium hydroxide (\ (\text {naoh}\)) and sulfuric acid (\ (\text {h}_ {2}\text {so}_ {4}\)). The titration formula calculates the concentration of an analyte by multiplying the normality (n) of the titrant by its volume (v). The reaction between kmno 4 and oxalic acid is a redox reaction and the titration. Titration Formula Class 12.
From mungfali.com
Acid Base Titration Method Titration Formula Class 12 Ammonium ferrous sulphate or ammonium iron (ii) sulphate is called mohr’s salt. The reaction between kmno 4 and oxalic acid is a redox reaction and the titration is therefore called a redox titration. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. It is an inorganic light green coloured. A titration can define. Titration Formula Class 12.